The MedMal Room: The $12 Million Misoprostol Case
When routine replaces reflection, consent becomes a courtroom exhibit. A $12 million verdict shows why forensic ethics belongs in every labor unit.
Preamble:
According to the American College of Obstetricians & Gynecologists (ACOG), informed consent should be obtained before any diagnostic or therapeutic procedure, ideally early enough in the clinical course to allow time for reflection and questions. It should also be revisited whenever new information arises or the treatment plan changes, ensuring that consent remains informed, voluntary, and current throughout care.
Should there always be an informed consent for induction of labor? When a physician prescribes a medication that has been deemed safe by a professional organization does that mean no informed consent is needed? Do patients have a reasonable expectation to be informed when a doctor recommends a medication whose manufacturer explicitly cautions against such use due to identified risks, and it has not been approved by the regulatory authorities?
The Case
A 38-year-old woman, pregnant for the first time, is admitted for induction of labor at 39 weeks. The reason: she’s 39 weeks pregnant. Not hypertensive. Not diabetic. Just term.
No informed consent for induction is obtained. No documented discussion of risks, alternatives, or the off-label nature of the medication.
She is supposed to have received 25 micrograms of misoprostol vaginally, a small white tablet better known by its brand name, Cytotec. For induction of labor at 39 weeks, hospitals often use low-dose vaginal misoprostol—typically 25 mcg every 4–6 hours, using a quarter of a 100 mcg tablet per dose. However, splitting a 100 mcg tablet into exact quarters is technically difficult, and studies show substantial dose variation when trying to divide tablets into four parts, even with a pill cutter or razor blade. This dose inaccuracy can lead to concerns about both efficacy and safety, so many institutions recommend using commercially available 25 mcg preparations whenever possible or provide pharmacy assistance for accurate splitting when lower strengths are unavailable. Clinical protocols often acknowledge these challenges and emphasize the need for careful tablet handling, standardized procedures, and close clinical supervision during the induction process.
After the first dose, contractions begin: every 3 to 4 minutes, fetal heart rate reassuring. Four hours later, a second dose is given. Within an hour, she develops tachysystole—five to six contractions in ten minutes, with late decelerations on the monitor.
For two hours, the pattern worsens. Then a stat cesarean is performed.
The newborn’s Apgars are 1 and 2, followed by neonatal seizures and long-term developmental delay. The family sues.
After a two-week trial, the jury awards $12 million. Their reasoning is simple: the patient was never properly informed, never warned about misoprostol’s risks, and never told that the drug was not FDA-approved for induction of labor.
The Forensic Ethics of Induction
This case isn’t about a bad doctor. It’s about an inadequate imperfect system—one that allows routine to replace reflection.
Induction of labor has become so common that its ethical gravity has been diluted. “Elective induction” at term now accounts for a growing proportion of deliveries in U.S. hospitals, often driven by convenience, scheduling, or soft indications like “maternal age” or “favorable cervix.”
But common doesn’t mean benign. Every induction is a medical intervention with physiologic, pharmacologic, and ethical dimensions.
In this case, these issues stand out:
No informed consent. An informed consent is required for an induction. There was no signed or documented consent, no record of explaining the risks of uterine hyperstimulation, fetal distress, or cesarean.
Use of an off-label drug without disclosure. The American College of ObGyn (ACOG) feels it’s safe to use Misoprostol. Using it is ethically permissible only when the patient is informed and the rationale is documented. In this case she did not give informed consent. Misoprostol is widely used in obstetrics, but it is not FDA-approved for induction of labor. The drug’s labeling specifically warns against this use.
Too much time passed watching a concerning tracing: Two hours is too long with a truly concerning tracing before a cesarean delivery is done.
The Legal Lens
The courtroom did not punish the physician for bad luck. It punished the system for bad consent.
The plaintiffs argued that the mother never received an informed consent. She never fully agreed to an off-label medication, that she was not warned of hyperstimulation or fetal distress, and that no alternatives, such as oxytocin or mechanical ripening, were discussed.
Expert testimony reinforced that induction of labor and off-label use require explicit informed consent, especially when safer or approved alternatives exist.
The defense argued that misoprostol was standard practice, used in most hospitals, and is ACOG “approved”. The jury wasn’t convinced. “Standard” doesn’t excuse silence. Approval by ACOG still does not mean informed consent is not needed. Jurors repeatedly cited the lack of written consent and the absence of documentation as evidence of negligence. No mention of indication, no evidence of discussion. Just routine words masking a chain of preventable events.
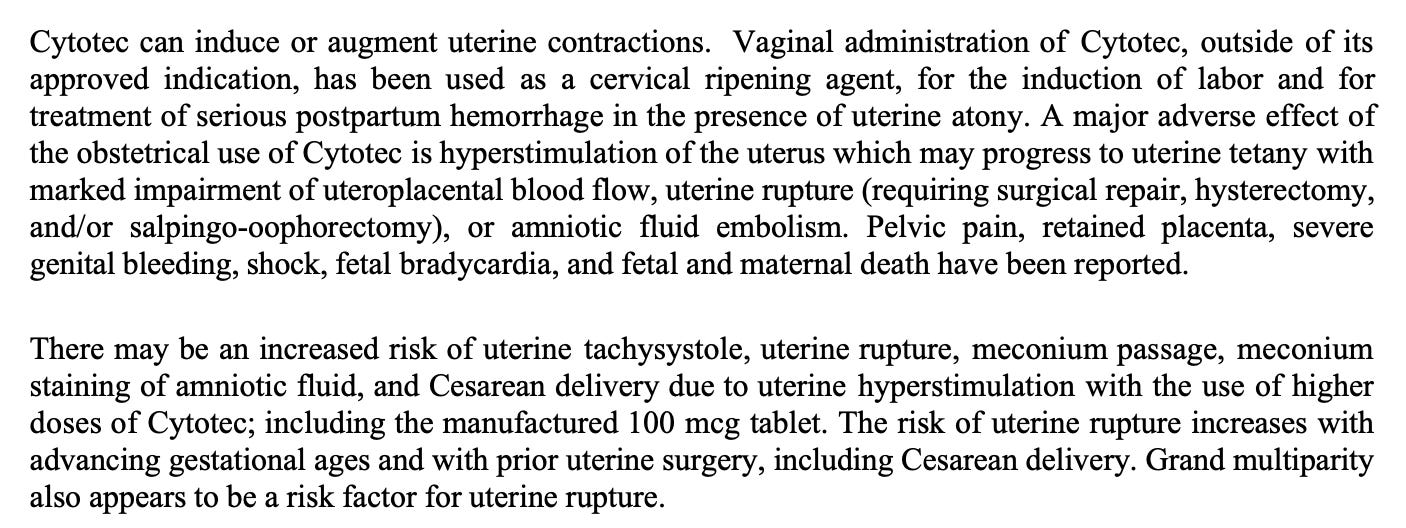
From The Cytotec insert:
https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/19268slr037.pdf
Complications and Adverse Effects of Cytotec (Misoprostol) in Obstetric Use
Uterine hyperstimulation (tachysystole) which can progress to uterine tetany
Impaired uteroplacental blood flow, leading to fetal hypoxia or bradycardia
Uterine rupture, sometimes requiring surgical repair, hysterectomy, and/or salpingo-oophorectomy
Amniotic fluid embolism
Severe pelvic pain
Retained placenta
Severe genital or postpartum bleeding leading to hemorrhagic shock
Fetal bradycardia and fetal death
Maternal death (rare but reported)
Meconium passage and meconium-stained amniotic fluid associated with hyperstimulation
Increased rate of Cesarean delivery due to uterine hyperstimulation or non-reassuring fetal status
Increased risk of uterine rupture with:
Advancing gestational age
Prior uterine surgery (especially prior Cesarean section)
Grand multiparity
Unestablished effects on the long-term growth, development, or functional maturation of the child
Unknown impact on the need for instrumental delivery (e.g., forceps, vacuum) or other obstetric interventions
Induction of Labor At 39 weeks
The widespread adoption of “routine” induction of labor at 39 weeks in low-risk nulliparous women, largely influenced by the ARRIVE trial’s findings of reduced cesarean delivery rates (18.6% vs 22.2%), represents a significant recent shift in obstetric practice that warrants careful scrutiny.
A critical limitation in the existing evidence base, including ARRIVE and subsequent supporting studies, is the frequent failure to stratify outcomes by induction method, despite substantial pharmacologic and physiologic differences between agents.
Misoprostol, oxytocin, dinoprostone, and mechanical methods like transcervical balloon catheters each carry distinct risk-benefit profiles, with varying rates of tachysystole, uterine rupture, failed induction, and neonatal outcomes.
The heterogeneity in induction protocols across institutions, combined with patient-specific factors like cervical favorability and parity, means that the aggregate cesarean reduction reported in these trials may not translate uniformly across all induction methods.
When counseling patients about elective induction at 39 weeks, of for that matter for any induction, informed consent must be obtained.
Patient autonomy requires that the specific induction agent and protocol should be explicitly discussed, as the safety profile and likelihood of vaginal delivery can differ markedly between a low-dose oxytocin protocol versus misoprostol administration, particularly in women with an unfavorable cervix. The absence of method-specific outcome data in most large trials represents a significant gap in our evidence base that directly impacts informed consent and clinical decision-making.
The Clinical Reality
Misoprostol, a prostaglandin E1 analogue, is effective at ripening the cervix and inducing contractions. It is cheap, stable at room temperature, and has transformed obstetric practice globally. And ACOG approves its use.
But it is also potent and unpredictable. The same microgram dose that softens a cervix can also hyperstimulate a uterus, cutting off fetal oxygenation within minutes.
When tachysystole occurs, the response must be immediate: remove residual drug, administer tocolytics, reposition the patient, and evaluate fetal status. In this case, observation continued for two hours, a delay that likely turned reversible hypoxia into irreversible injury.
The Ethical Chain of Prevention
Several breaks in the chain of ethical and safety practices are visible here:
Indication:
Inductions should follow a documented medical rationale, consistent with ACOG guidelines. “Advanced maternal age” at 38 is not itself an emergency.Consent:
True informed consent requires explaining the why, how, risks, alternatives, and off-label status. Consent must be documented, preferably written and signed.Monitoring:
Tachysystole with fetal heart rate changes requires immediate intervention, not extended observation. Protocols should define clear thresholds for calling obstetric anesthesia and performing cesarean.Protocol clarity:
Hospitals should have standardized induction order sets, explicitly naming drugs, dosages, indications, contraindications, and consent requirements.
Forensic Ethics in Practice
This case illustrates a principle I call forensic ethics, the study of medical conduct through its legal aftermath.
Forensic ethics asks not only what happened but why it was predictable. It examines how everyday shortcuts, verbal consents, undocumented discussions, “routine” practices, create ethical blind spots that later become courtroom evidence.
In this view, malpractice is not just about deviation from standard of care. It’s about deviation from ethical transparency. When patients are not informed, they cannot consent. When clinicians assume understanding, they erode trust. When systems fail to require documentation, they invite litigation.
System-Level Solutions
Hospitals can prevent similar cases through three simple but powerful reforms:
Mandatory Consent for Induction, optimally written.
Every induction, regardless of method, should require a consent form detailing benefits risks (eg, hyperstimulation, cesarean, fetal distress), and alternatives. There are no legal requirements that a patient must be informed that a drug is being used off-label, however there are ethical arguments that as part of the informed consent it should be disclosed. This protects both patients and clinicians.Induction Protocols by Indication.
Develop standardized pathways: postdates, hypertensive disorders, diabetes, advanced maternal age, etc. Each with recommended agents, monitoring frequency, and escalation steps.Real-Time Safety Checks.
Create a “tachysystole response bundle”: if >5 contractions/10 min with decelerations, the nurse and provider must initiate resuscitation such as tocolysis and notify charge staff within 5 minutes. Document all steps. Watch carefully the fetal heart rate.Audit and Feedback.
Chart audits of induction cases, focusing on consent completeness, indication, and timing of intervention, should be part of hospital safety dashboards.
The Lesson
The tragedy here was not simply that a baby was injured. It was that everyone believed they were doing what was normal.
No one paused to ask: Does the patient know what we’re using? Did we fully explain potential risks and that misoprostol is off-label? Are we sure this induction is necessary today?
Ethical practice is not about predicting outcomes, it’s about preventing foreseeable harm. And nothing in this case was unforeseeable.
When medicine becomes routine, ethics must become intentional.
Reflection
If the profession learns anything from this verdict, let it be this:
The price of informed consent is time, not millions.
Would your hospital’s induction policy stand up to this courtroom?
If not, it’s time to rewrite it. Before a jury does.
A Personal Disclaimer:
When I was in charge of two large L&D units between 1987 and 2018 our units did not allow induction of labor with Misoprostol for several reasons, including that the staff believed that potential advantages did not justify risks. Non-FDA approval also played a role.
The consequence was that our cesarean deliveries for “fetal distress” with inductions dropped (at one point the NICU complained about reduced admissions), we reduced liability expenses, there were no amniotic fluid embolisms while patients were being induced, and interestingly, our cesarean delivery rates decreased.
https://pubmed.ncbi.nlm.nih.gov/21284964/




I received some feedback and would like to respond:
After all, this was a jury court case that is being described, and we cannot perform medicine in isolation.
I appreciate this thoughtful discussion. While I completely agree that the primary clinical failure was the delayed response to two hours of tachysystole with Category II/III tracings, these issues that are described are not mutually exclusive. The standard of care for management doesn't negate our ethical obligation to discuss off-label medication use during the consent process—this is particularly salient when adverse outcomes occur and documentation becomes scrutinized. ACOG's evidence-based recommendation for misoprostol's efficacy doesn't eliminate the medicolegal reality that off-label use requires disclosure, especially given that many patients are unaware their induction agent lacks FDA approval for this indication. My intent was not to "fear monger" about a medication that is being used regularly, but to highlight how gaps in informed consent documentation can compound liability when clinical management falters, which serves our patients and our specialty better than dismissing the consent conversation entirely.
Great article. Really eye opening for me how we can become a bit complacent about these issues when they become routine