Doctor, are my nausea meds vegan?” Hyperemesis, ingredients, and informed choice
What options doe vegans or vegetarians have as medications for hyperemesis gravidarum
She is 12 weeks pregnant, dehydrated, and scared. She is also vegan. “Doctor, can I take this if it has gelatin or insect shellac?” she asks, clutching a prescription for anti-nausea medicine. The question is not trivial. For a patient with hyperemesis gravidarum, skipping treatment can mean IV fluids, weight loss, and hospital admission.
What the issue really is
Hyperemesis gravidarum is severe, persistent nausea and vomiting in pregnancy. It threatens hydration, nutrition, and mental health. The ethics are simple to state and hard to practice: relieve suffering, protect mother and fetus, respect values. The catch is that medications can include animal-derived ingredients in capsules, coatings, or excipients. This is rarely highlighted on the label. Patients with vegan, vegetarian, halal, or kosher needs deserve clarity and options without delay.
Dietary beliefs and their medical implications
Before discussing medications, it helps to understand what “vegan” and “vegetarian” actually mean.
Vegetarianism generally excludes meat, poultry, and fish, but many vegetarians still consume dairy and eggs.
Veganism goes further, avoiding all animal-derived products, including milk, eggs, honey, gelatin, and shellac. Motivations vary: some people adopt these diets for health or environmental reasons, others for ethical or religious convictions. Globally, about 5–8% of people identify as vegetarian and roughly 1–2% as vegan, with higher rates in younger and urban populations.
Religions such as Hinduism, Buddhism, and Jainism often encourage vegetarian eating, while Judaism and Islam have their own dietary laws.
Kosher rules forbid pork and require meat to be slaughtered in a specific ritual manner, while Halal rules similarly prohibit pork and restrict alcohol or animal products not slaughtered according to Islamic law.
Both traditions also extend these principles to medicines: capsules made from porcine gelatin, alcohol-based elixirs, or non-certified ingredients may be considered non-permissible. For patients who follow these beliefs, the ethical concern does not end with food—it extends to medications, supplements, and even capsule coatings.
Common animal-derived components to know
Gelatin capsules: often bovine or porcine. Seen in some doxylamine-pyridoxine products and various softgels.
Shellac, E904: a glazing agent from lac insects, used in some enteric or shiny film coatings.
Lactose: milk-derived filler in many tablets, including some ondansetron, metoclopramide, and promethazine.
Magnesium stearate, stearic acid: may be animal or plant sourced, varies by manufacturer.
Glycerin: in liquids or softgels, can be animal or synthetic.
Formulations vary widely by brand and country. The same active drug may come in vegan and non-vegan versions.
How to identify animal ingredients in medications
Finding out exactly what is inside a pill is harder than it should be. Active ingredients are always listed, but inactive ingredients (excipients) such as gelatin, lactose, or shellac may not appear clearly on the box or leaflet.
Manufacturers must disclose them in the official product monograph or package insert, but these documents can be difficult to access or interpret. Some over-the-counter brands list the capsule composition (for example, “gelatin capsule”) while many prescription drugs do not specify whether the source is plant or animal. Pharmacies and hospital formularies often use electronic databases that identify excipients and, when known, their origin.
However, regulations do not currently require pharmaceutical companies to state whether an ingredient is animal, plant, or synthetic, unless it affects safety, such as an allergy risk.
This lack of transparency leaves both patients and clinicians guessing, especially when the same medication from different manufacturers may vary in composition. Checking the detailed insert, consulting a pharmacist, or contacting the manufacturer directly are often the only ways to confirm if a drug is truly vegan, halal, kosher, or vegetarian-friendly.
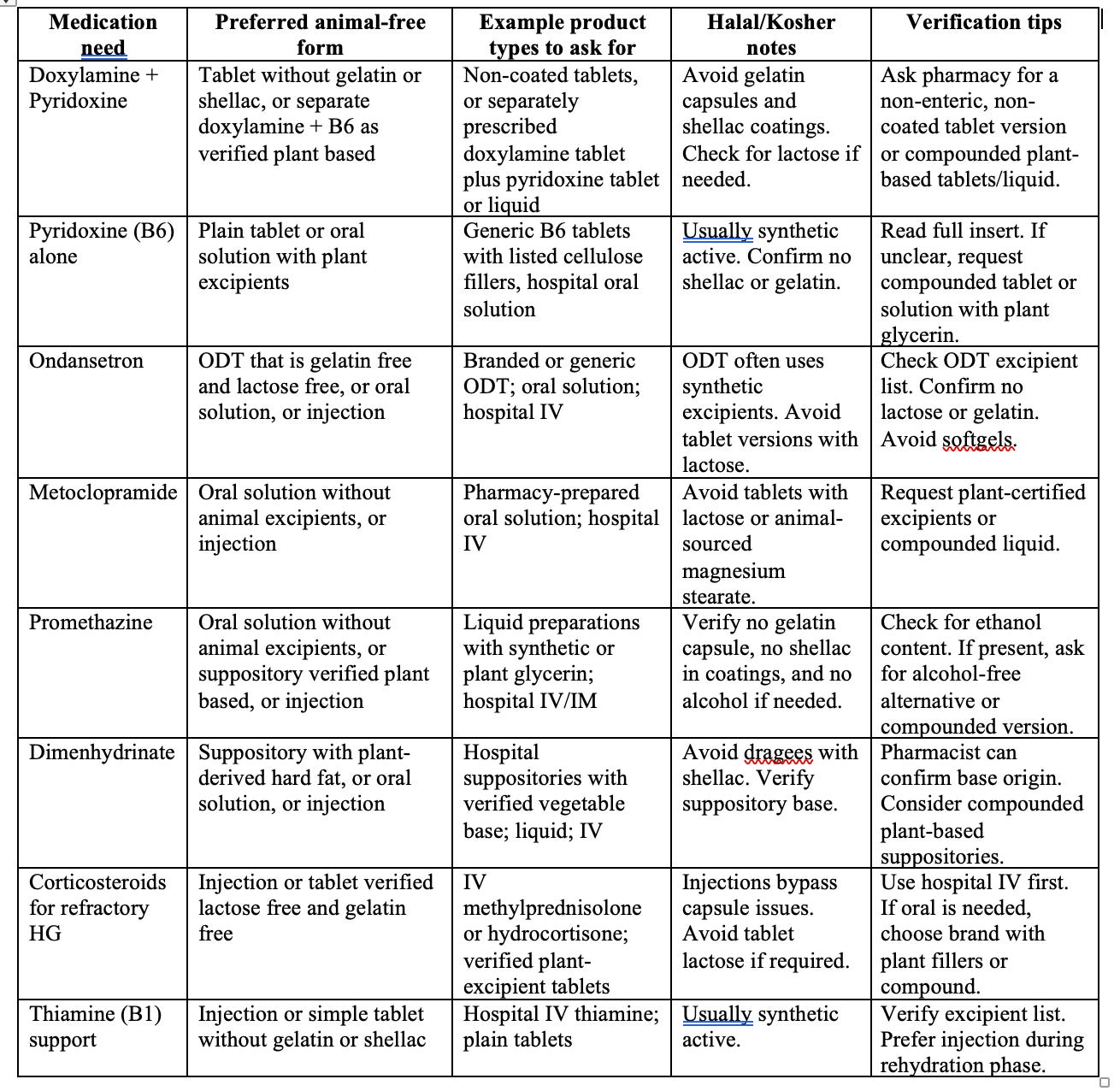
Medication-specific notes patients ask about
Pyridoxine, vitamin B6: active is typically synthetic. Check the capsule and excipients. Tablets or solutions can be animal-free.
Doxylamine-pyridoxine combos: some products use gelatin capsules or shellac-coated tablets.
Ondansetron: many standard tablets contain lactose. ODT forms differ; branded ODT often contains aspartame, usually no animal products. Always verify excipients.
Metoclopramide: tablets frequently include lactose. Solutions and injections can avoid animal ingredients.
Promethazine: tablets may include lactose or shellac, suppositories vary.
Corticosteroids for refractory cases, such as methylprednisolone or prednisone: some tablets have lactose, others use plant fillers. Injections avoid capsule issues.
Practical playbook for clinicians and families
1) Ask, listen, and name the value: “Your vegan commitment matters. We will treat your hyperemesis and honor your values.”
2) Check the exact product, not only the drug name. Brand, dosage form, and country matter. Request the official product insert or pharmacy database entry that lists excipients.
3) Use pharmacy as a partner. Hospital and retail pharmacists can identify vegan or vegetarian formulations and document sources of magnesium stearate, stearic acid, and glycerin. Many hospital pharmacies keep an animal-free list.
4) Choose forms that avoid common pitfalls.
Prefer ODT ondansetron when a lactose-free, gelatin-free option is confirmed.
Consider tablets with verified plant excipients or liquid solutions where glycerin is synthetic or plant based.
Injections in hospital care bypass capsule and coating issues when needed.
5) Compounding when necessary.
If a suitable commercial product does not exist, a compounding pharmacy can prepare animal-free tablets or liquids with plant-based or synthetic excipients. This is crucial for sustained outpatient therapy.
6) Give a stepwise, evidence-based regimen.
Start with pyridoxine plus a tolerated antihistamine, verify excipients.
Add ondansetron using a verified ODT or solution when available.
Consider metoclopramide with an animal-free formulation if ondansetron is insufficient.
Use promethazine or dimenhydrinate where a confirmed vegan form exists.
For refractory cases, discuss short steroid courses, checking excipients, balancing benefits and risks.
Combine with IV fluids, thiamine, and electrolyte support when indicated.
7) Document consent with ingredients.
Write the agreed product, manufacturer, and the excipient check in the chart. This respects autonomy and avoids delays if the pharmacy changes brands.
8) Offer supportive non-drug measures that align with values.
Small frequent meals, ginger, vitamin B1 when dehydrated risk exists, acupressure bands, sleep optimization, and trigger avoidance. These do not replace antiemetics in hyperemesis, but they can help.
What is overlooked
We talk a lot about active drugs and dose, less about the capsule shell and coating. For many patients, the excipient is the moral line. Clear communication prevents dangerous “all or nothing” decisions. Offering a verified plant-based alternative often restores trust and keeps treatment on track.
Practical takeaways
Verify the specific product’s excipients every time.
Partner with pharmacy and consider compounding early.
Injections and ODT can avoid common animal ingredients.
Never advise “just skip it” for severe hyperemesis. Treat the illness and respect the value.
A new ally: using AI to decode ingredients
Artificial intelligence can now help patients and clinicians check medications more easily. By taking a clear photo of the package insert or ingredient list and uploading it into a trusted AI assistant, you can ask a simple prompt such as:
“Analyze this medication label and tell me if any ingredients are animal-derived or not vegan.”
AI can quickly scan for substances like gelatin, shellac, lactose, or magnesium stearate and explain their likely sources. It is not a substitute for a pharmacist, but it can be a useful first step toward informed, value-based decisions about medication use.
Evidence-based summary of hyperemesis gravidarum medications
Here is a detailed, evidence-based summary of hyperemesis gravidarum medications known or reported to contain animal-derived ingredients. Formulations vary by manufacturer and country, so these are representative examples based on official product monographs and excipient listings.
1. Doxylamine-Pyridoxine Combinations
Products: Diclectin, Cariban, Xonvea
Gelatin: Bovine or porcine, used in capsule shells (Cariban, Xonvea).
Schellack (Shellac, E904): Derived from lac insects; used as coating in Diclectin/Cariban delayed-release tablets.
Titanium dioxide: Mineral, not animal, but often paired with shellac.
Lactose monohydrate: Milk-derived filler in tablets.
Magnesium stearate: May be animal or plant-sourced, manufacturer dependent.
2. Ondansetron (Zofran, generics)
Lactose monohydrate: Common inactive filler in standard tablets.
Magnesium stearate: Possible animal origin unless plant-certified.
Gelatin: Present in some softgel or capsule forms, not in ODT (Zydis) versions.
Aspartame (in ODT): Synthetic, non-animal.
→ Zofran ODT/Zydis is generally the most vegan-compatible form.
3. Metoclopramide (MCP, Paspertin, Reglan)
Lactose monohydrate: In most tablet formulations.
Magnesium stearate: Animal or plant origin depending on supplier.
Gelatin: Absent in standard tablets, but may appear in capsules or compounded liquids.
4. Dimenhydrinate (Vomex A, Dramamine)
Schellack (Shellac): Used in coating of Vomex A dragees.
Lactose monohydrate: Often present in tablets.
Magnesium stearate: Possible animal source.
Gelatin: In some capsule formulations (especially softgels).
→ Vomex A suppositories usually contain plant-derived hard fat but should be verified per batch.
5. Promethazine (Phenergan, Generika)
Lactose monohydrate: Common filler in tablets.
Magnesium stearate: May be animal-derived.
Gelatin: Used in capsule shells of some formulations.
Shellac: May appear in coated tablets.
6. Corticosteroids (Methylprednisolone, Prednisone, Hydrocortisone)
Lactose monohydrate: In many tablet forms.
Magnesium stearate: May be animal or plant sourced.
Gelatin: Occasionally used in capsule formulations.
→ Injectable forms usually avoid animal excipients.
7. Vitamin B6 (Pyridoxine) and B1 (Thiamine)
Gelatin: In some capsule supplements.
Shellac: In coated tablets.
Lactose: In several over-the-counter brands.
→ Pure tablet or liquid B6 formulations are typically synthetic and animal-free.
The ethical question is simple: will we treat suffering while honoring conscience. The clinical answer requires a few extra phone calls and a pharmacy partnership. That is a small price to keep both the patient’s health and her values intact
Hyperemesis needs fast, effective care. With a quick excipient check and pharmacy support, we can honor vegan or religious beliefs without compromising safety.